Lithium[]
Lithium is a soft silvery reactive metal and is the lightest solid element. It is
tric disorders.Its symbol is Li, and it has the atomic number 3. The name Lithium is from the Greek word lithos, meaning stone. (1)

Lithium - Periodic Table of Videos-0
Lithium Overview
Where Does Lithium Come From?[]

Lithium Reserves (3)
Lithium can be found in 5 different continents. South America claims 48% of the world's Lithium reserves. With less than half of South America's amount of Lithium, Australasia (Australia and the surrounding islands) comes in next with 23% of the world's lithium reserves. North American comes in a close third with 20% of the world's Lithium. Europe and Africa trail in with 6% and 3% respectively.
Lithium is not found naturally in its elemental state, instead it is found in minerals known as spodumene (LiAl(SiO3)2) or petalite/castorite (LiAlSi4O10) and in salt water.
Lithium Extraction from Mineral Form[]
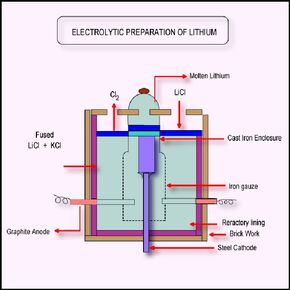
Lithium Extraction [14]?-02-0039
- The minerals containing Lithium are heated up to 1200K.
- The minerals are then easily crumbled at this temperature.
- The minerals are combined with sulfuric acid and sodium carbonate which causes the Aluminum and Iron to precipitate from the ore.
- Sodium carbonate is added to the Lithium products which causes the Lithium to precipitate out in the form of Lithium carbonate.
- Hydrochloric acid is added to the Lithium carbonate to form Lithium chloride.(4)
Lithium Extraction from Brine[]
Lithium can also be obtained by evaporation:
- 87% of the world's current lithium is produced from this method.
- This process is time-consuming, but is also inexpensive compared to other methods.
- The Salt-Rich waters are pumped from the ground and start to evaporate through solar energy. This process can take several months.
- Firstly, potassium is harvested.
- Then when the lithium reaches a good concentration, it is harvested and brought to a plant.
- Unwanted waste is filtered out, then it is treated with sodium carbonate, to create lithium carbonate.
- Finally, the unwanted waste is pumped back into the ground.

Lithium from brine (10)
Uses for Lithium []
There are eight main technologies that lithium and lithium compounds are used for:
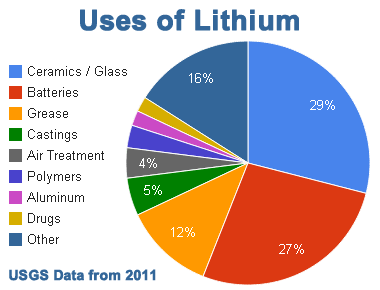
(4)
1. Ceramics/Glass: Lithium oxide is

(14) Pottery with a lithium-based glaze
used for processing silica. It reduces the melting point and viscosity of the material, which produces glaze that is used in ovenware. 2.Batteries: Lithium is used in lithium-ion batteries because it has a high

(16) Lithium Ion Batteries
electrochemical potential and a single cell can generate 3 volts, as compared to other elements such as zinc-carbon cells which can only generate 1.5 volts.
Also, lithium ion batteries have been used and are currently used in Boeing 787s. However, a few of these batteries short-circuted in Japan Airlines' 787s. While the lithium ion batteries are currently being tested around the globe to determine the cause of the problem, some airlines have returned their 787s to service. (13)

(17) Lithium Grease
3. Grease: When lithium hydroxide is heated with a fat, a soap made of lithium stearate is produced. This soap is used to thicken oils and to manufacture all-purpose, high-temperature lubricating greases.

(6)
4. Castings: Metallic lithium aids the fusing of metals during welding and soldering and prevents oxides from forming by absorbing impurities. Alloys of lithium with aluminium, cadmium, copper and manganese are used to make high-performance aircraft parts. Some lithium compounds are also used as oxidizers in red fireworks and flares.

(18) Structure of Lithium Peroxide
5. Air Treatment: Lithium hydroxide and lithium peroxide are used for carbon dioxide removal and air purification in confined areas, such as aboard spacecraft and submarines. The use of lithium peroxide also releases oxygen gas, so it is used to produce oxygen in these confined areas as well.

(19) Organolithium compounds are useful in organic synthesis.
6. Polymers: Alkyl lithium compounds are used as catalysts in the polymer industry. Lithium metal and alkyl halides are used to prepare organolithium compounds, which are used as strong bases for the production of fine chemicals and for the formation of carbon-carbon bonds in organic synthesis.

(20) Lithium alloys are used in space shuttles.
7. Aluminum: Metallic lithium and lithium aluminum hydride are used as high energy additives to rocket propellants, which are utilized by the military.
8. Drugs: The standard medications used in the treatment of bipolar disorder are

(21) Lithium is used in some drugs.
lithium compounds. It is also thought that lithium salts may help treat related disorders, such as schizoaffective disorder and cyclic major depression. (2)
Environmental effects[]
Lithium reacts easily in water and does not occur freely in nature due to its activity. Metallic lithium will react with nitrogen, oxygen, and water vapor in air. Consequently, the lithium surface becomes coated with a mixture of Lithium hydroxide (LiOH), Lithium carbonate (Li2CO3), and Lithium nitride (Li3N). Lithium hydroxide represents a potentially significant hazard because it is extremely corrosive.(22) Other concerns focus around the environmental effects of mining and extracting lithium, as with many other metals.
Supply and Demand[]
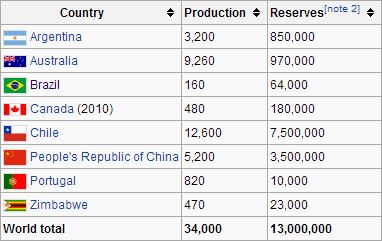
Lithium mine production and reserves in tonnes (2)
The two main sources of Lithium are brine lakes and salt pans, which produce the soluble salts Lithium carbonate and Lithium chloride, and a hard mineral called spodumene, which is a silicate or glass of Lithium and Aluminium. The main producers of Lithium from brine are Argentina, Chile, and more recently, Bolivia. Australia is the main producer of spodumene. The U.S. has one main source of Lithium brine in Nevada, and a small amount is put back into the market through recycling.
Significant apparent consumption of Lithium in the U.S. began in the 1950s, peaked in 1974, and has shown a slightly decreasing trend since 1974. In recent years, there has been a slight increase in consumption due to advances in technology that used Lithium batteries. It is forecast that if the production of hybrid and electric cars takes off, Lithium demand will spike.(11)
The Future of Lithium[]

(8)
It is expected that lithium batteries will be mass produced in the near future for hybrid, plug-in hybrid and electric vehicles using lithium batteries. The major automotive manufacturers which present the greatest potential for lithium demand are Toyota, Honda, Nissan, Renault, BYD, Mitsubishi, Hyundai, Ford, Chevrolet and GM. (7)
The United States Geological Survey predicts that the reserves contain 10 million tons of lithium. Energy expert Nick Butcher calculates that at current extraction rates, we have enough known reserves to last 300 years.
Sources[]
(1)"Lithium." Chambers 21st Century Dictionary. London: Chambers Harrap, 2001. Web. 22 April 2013.
(2) "Lithium." Wikipedia Wikimedia Foundation, 20 Apr. 2013. Web. 20 Apr. 2013.
(3) "About Lithium: A Vital Component of the Electron Economy." Australian Lithium N.p., n.d. Web. 22 Apr. 2013
(4)"Chemistry of Lithium - ChemWiki." Chemistry of Lithium - ChemWiki. N.p., n.d. Web. 22 Apr. 2013.
(5) "Spodumene." : Used as a Lithium Source Mineral and as a Gemstone. N.p., n.d. Web. 22 Apr. 2013.
(6) Cyberchemist. "Lithium Flame Color 2." Flickr. Yahoo!, 05 Oct. 2007. Web. 22 Apr. 2013.
(7) "Lithium." Lithium. N.p., n.d. Web. 22 Apr. 2013.
(8) "What Is the Plug-in Electric Vehicle Credit?" HowStuffWorks. N.p., n.d. Web. 22 Apr. 2013.
(9) "Peak Lithium: Death Blow For Electric Cars?" - Seeking Alpha. N.p., n.d. Web. 22 Apr. 2013.
(10) "Lithium Facts" New World Resource, N.p., N.p. Web. 24 Apr. 2013.
(11) " The Trouble with Lithium" Meridian National Research, December 2006. EV World. Web. 24 Apr. 2013
(12) "Lithium Demand Forecast" Tru Group, N.p., N.p. Web. 24 Apr. 2013.
(13) "NTSB Seeks More Dreamliner Lithium-ion Battery Tests." Widgets RSS. N.p., n.d. Web. 08 May 2013.
(14) Examville.com. "Extraction, Properties and Uses of Lithium." Scribd. N.p., n.d. Web. 08 May 2013.
(15) "Ceramics Piece #2." -Look Mom. N.p., n.d. Web. 08 May 2013.
(16) "The Revolution in Rechargeable Batteries: Lithium Iron Vs Lithium Ion." The Revolution in Rechargeable Batteries: Lithium Iron Vs Lithium Ion. N.p., n.d. Web. 08 May 2013.
(17) "Lith-Ease White Lithium Grease 16oz. or 454gm Tub." Lith-Ease White Lithium Grease 16oz. or 454gm Tub. N.p., n.d. Web. 08 May 2013.
(18) "Dilithium Peroxide." WebElements Periodic Table of the Elements. N.p., n.d. Web. 08 May 2013.
(19) "Organometallics - Addition to the Carbonyl Group." Organometallics - Addition to the Carbonyl Group. N.p., n.d. Web. 08 May 2013.
(20) "Light Combat Aircraft (LCA)." Light Combat Aircraft (LCA). N.p., n.d. Web. 08 May 2013.
(21) "Answers and Tips from 35,000 U.S. Doctors.No Waiting Room. FREE!" HealthTap. N.p., n.d. Web. 08 May 2013.
(22) "Lithium" www.lenntech.com/periodic/elements/li.htm, n.d. Web. 09 May 2013.

